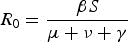The Origin & Drivers of Virus Variants
Viruses, such as SARS-CoV-2, continuously evolve as changes in the genetic code (mutations) occur as natural by-products of viral replication. Some viruses mutate extremely rapidly, as is the case for SARS-CoV-2, enabling rapid evolution of the virus genome.
However, mutation is not the goal of any pathogen. It is just the means to an end: to find evolutionary fitness. Understanding that end and how it is achieved is important, and is quite well understood, also mathematically.
A simplified example of the expression of pathogen fitness, R0.
Where fitness is the product of the rate at which new infections are caused by an infected host (βS) and the duration of infection (μ + ν + γ). In this formulation, β is the transmission rate, S is the density of susceptibles, γ is the rate at which an infected host clears the pathogen, μ is the background mortality rate and ν is the mortality rate due to infection, often referred to as ‘virulence’.
These dynamics can become more complicated when people are being vaccinated, drastically reducing S. In principle this will reduce fitness. However, the impact of fitness reduction depends on how many people in a population are vaccinated. When only a low percentage of a population is vaccinated, the virus can still freely replicate. Crucially, it can also infect vaccinated people, and, on occasion, still spread from these individuals (because they will experience only mild symptoms). This way the virus can “learn” how to cope with vaccine induced immunity thereby restoring fitness. In fact, areas where vaccination is lagging are the perfect testbed for viruses to find a way around vaccines. This is why it is so important to vaccinate the whole global population properly during a pandemic.
However, this might not all be bad news. When natural or vaccine- induced immunity lowers S, theory suggests a reduced virulence (and a higher transmission rate). Something we now see with the Omicron variant of SARS-CoV-2. However, the interplay between pathogens and their hosts is much more complex than described here, and continuously evolving. In any case, the dynamics of host-pathogen interactions are always a bumpy ride, which might even include the occasional occurrence of increased virulence.
In most cases there will at least be immune evasion. Also, because fitness of pathogens can be shaped by evolutionary trade-offs at the individual scale. In a direct interplay with the individual host, there is a drive to find the optimal solution to temporary evasion of the immune system and spread to other hosts. These confrontations tend to be brief, mostly ending in neutralisation of the virus. However, in people with inadequate immune function where a virus is not efficiently cleared, it can persist, and mutate, for months. Importantly, in an environment with some immunological push-back, it can develop into a variant that can circumvent certain aspects of immunity. In fact, it is now clear that this significantly contributes to the emergence of new variants.
In any case, although mutations occur frequently, they only become ‘selected’ when they confer an advantage to the virus (on the population level). When a virus obtains only limited modifications to its genetic make-up, it is referred to as a variant. However, if the genetic mutations are more plentiful, resulting in significantly different physical attributes for the virus, we refer to this as a new strain.
Once a mutation occurs, a virus is changed. This change is either going to be an advantage to the virus, enabling it to thrive and/or survive more easily in its environment, or will be a disadvantage, by limiting replication, transmission, or even survival. Lastly, a mutation may be neutral, conferring neither an advantage nor a disadvantage. The process by which a virus or an organism is selected for or against by its environment is a process called natural selection. If the mutation confers an advantage with respect to viral replication, transmission, or escape from immunity, the mutated sequence then spreads within a population; if the mutation confers a disadvantage, the mutated sequence reduces in prevalence. Furthermore, the frequency of mutations also varies due to chance events. For instance, a “founder effect” occurs when a selected number of individual viruses establish a new population during transmission. By the "founder effect," a virus variant might become the most prevalent virus simply by chance, i.e. being the only variant within the new population, instead of because of a significant advantage. The high mutation rates of viruses, coupled with short generation times and large population sizes, allow viruses to rapidly evolve and adapt to new and changing environments.
Since the beginning of the COVID-19 pandemic, SARS-CoV-2 has mutated, and variants have started spreading rapidly across the world, with many more variants expected to develop. The variant containing the D614G mutation has become the wild-type (predominant) form of SARS-CoV-2 worldwide since mid-2020. The mutation likely increased the ability of the virus to bind the human ACE2 receptor, resulting in higher infectivity and transmission rates. However, variants can also become extinct due to natural selection.
The emergence of the latest SARS-CoV-2 variant, Omicron, is therefore expected to be only one of many in a line for this virus. However, something has changed profoundly with the rise of Omicron that did not occur with previous variants. Even though it is again more transmissible compared to previous variants, it is also less virulent (causes less disease). As mentioned above, it is possible that Omicron is the result of an attempt to increase fitness in a vaccinated world. This was partly expected, but mostly hoped for. However, this will not be the end of the (variant) line for SARS-CoV-2. And it is by no means a certainty that future variants will also be less virulent.
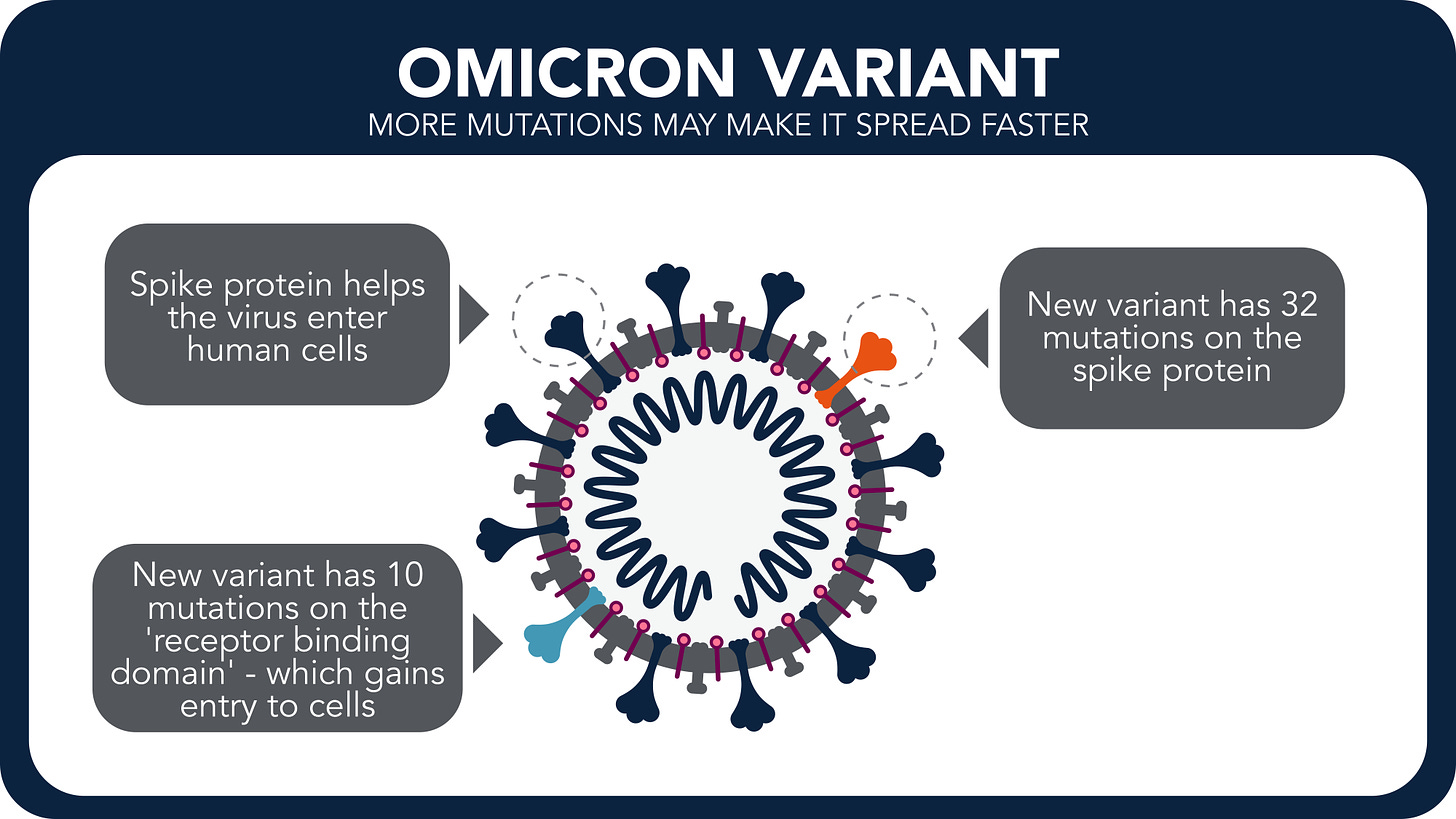
The Omicron variant carries over 50 mutations, including 32 mutations on the spike protein (see figure). Many of which are found in the Receptor Binding Domain (RBD), the regions of the spike trimer which interacts with the ACE2-receptor. The rest of the mutations on spike are expectedly involved in immune evasion. For more information about the SARS-CoV-2 infection process, please click here.
The World Health Organization (WHO) applied a classification strategy for (emerging) SARS-CoV-2 variants, where a variant is designated a Variant of Concern (VOC) when it has a clinical or public health significance that affects one or more of the following:
· Disease spread (transmission)
· Severity of the disease
· Diagnostic testing
· Vaccine and treatment efficacy
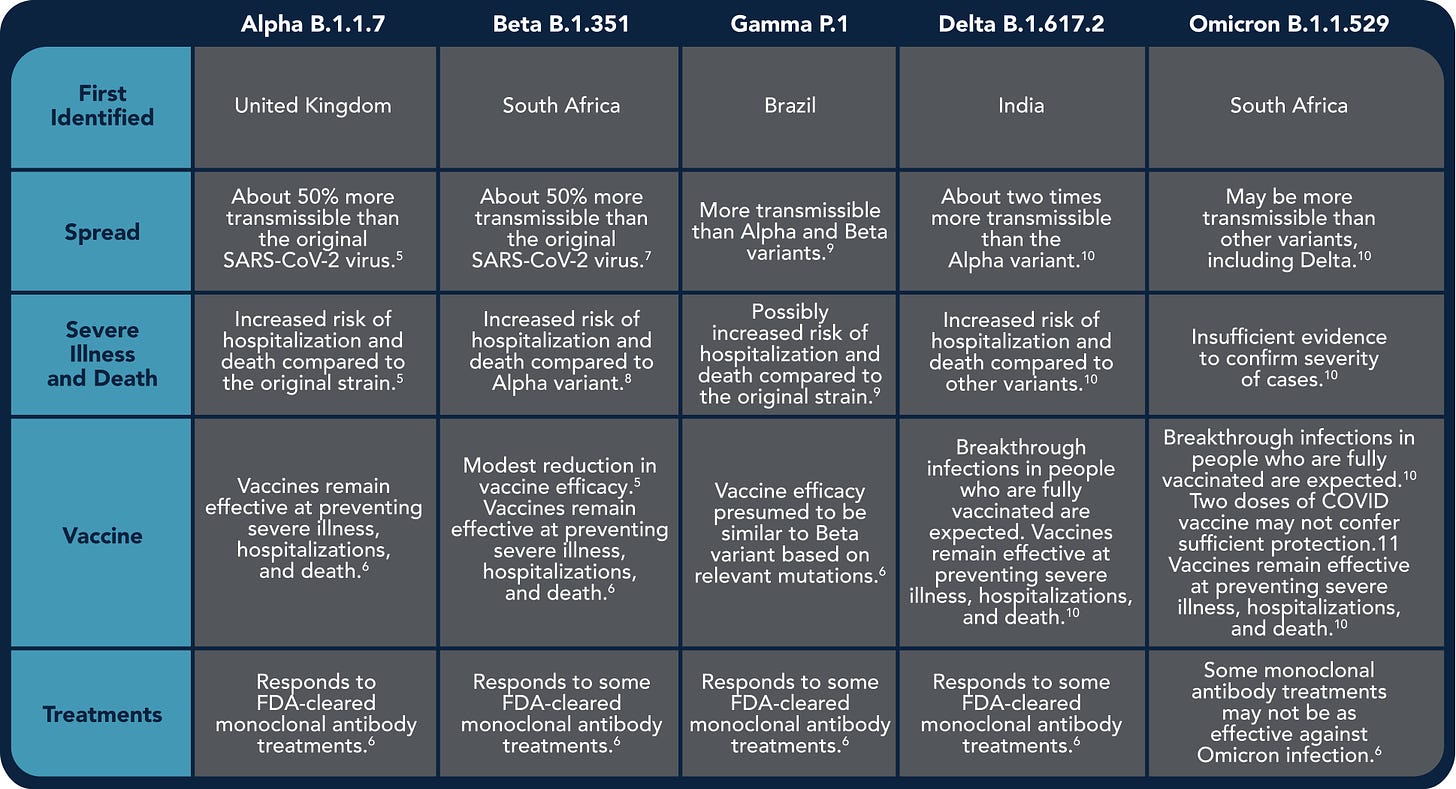
The Omicron variant was designated a VOC by the World Health Organization (WHO) in November of 2021. The following VOC’s of SARS-CoV-2 have been documented to date.
The following information expands on what we know about the Omicron variant that makes it a Variant of Concern.
Transmissibility
Some of the mutations identified in Omicron are also found in other VOCs. However, current evidence suggests that the combined changes to the spike protein of Omicron allow it to bind more tightly to ACE-2 and TMPRSS2, thereby enhancing its transmissibility.
Disease Severity
Currently, it is clear that infection with the Omicron variant is associated with less severe disease, compared to previous variants. Most patients infected with Omicron are either asymptomatic or report only mild symptoms. However, the impact of Omicron on disease severity in vulnerable populations is still unknown.
Diagnostic Assays
Current molecular and antigen tests for COVID are expected to be able to detect Omicron. However, early data suggests that the antigen tests may not reliably detect the Omicron variant in the first few days of infection.
Vaccine and treatment efficacy
Currently approved COVID-19 vaccines are based on the original (pre-alpha) SARS-CoV-2 spike protein. The mutations in the Omicron variant of SARS-CoV-2 induce some escape from antibodies raised against this original spike. Therefore, breakthrough infections of Omicron in vaccinated people are reported worldwide. However, initial reports suggest that cytotoxic T-cell immunity remains intact. Therefore, the existing vaccines still offer reasonable protection against severe disease. However, vaccines are not (and never were) 100% effective. And because Omicron is so much more transmissible, many people could end up in the hospital anyway. This is why alternatives to vaccination are more necessary than ever.
References:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4873896/
https://www.nature.com/articles/s41594-020-0469-6
https://apnews.com/article/coronavirus-pandemic-science-health-pandemics-dc99bc9f769dd6d7cb669e3d185c6261
Genetic Variants of SARS-CoV-2—What Do They Mean? JAMA. 2021;325(6):529–531. doi:10.1001/jama.2020.27124
World Health Organization. The effects of virus variants on COVID-19 vaccines [Internet].
Geneva: World Health Organization; 2021 Mar 1 [cited 2021 Dec 18]. Available from: https://www.who.int/news-room/feature-stories/detail/the-effects-of-virus-variants-on-covid-19-vaccines
Centers for Disease Control and Prevention and the Infectious Diseases Society of America.
Available from:
World Health Organization. Tracking SARS-CoV-2 variants [Internet]. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
Adi Stern, Raul Andino, Chapter 17 - Viral Evolution: It Is All About Mutations, Editor(s): Michael G. Katze, Marcus J. Korth, G. Lynn Law, Neal Nathanson, Viral Pathogenesis (Third Edition),
Academic Press, 2016, Pages 233-240, ISBN 9780128009642, https://doi.org/10.1016/B978-0-12-800964-2.00017-3.
Harvey, W.T., Carabelli, A.M., Jackson, B. et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19, 409–424 (2021). https://doi.org/10.1038/s41579-021-00573-0
Lauring AS, Hodcroft EB. Genetic Variants of SARS-CoV-2—What Do They Mean? JAMA. 2021;325(6):529–531. doi:10.1001/jama.2020.27124
Grubaugh ND, Petrone ME, Holmes EC. Why we shouldn’t worry when a virus mutates during disease outbreaks. Nat Microbiol. 2020;5:529-530
Baric RS. Emergence of a Highly Fit SARS-CoV-2 Variant. N Engl J Med. 2020;383:2684–2686
Burkholz S, Pokhrel S, Kraemer BR, Mochly-Rosen D, Carback RT 3rd, Hodge T, Harris P, Ciotlos S, Wang L, Herst CV, Rubsamen R. Paired SARS-CoV-2 spike protein mutations observed during ongoing SARS-CoV-2 viral transfer from humans to minks and back to humans. Infect Genet Evol. 2021 Sep;93:104897. doi: 10.1016/j.meegid.2021.104897. Epub 2021 May 7. PMID: 33971305; PMCID: PMC8103774.
Otto, S. P., Day, T., Arino, J., Colijn, C., Dushoff, J., Li, M., Mechai, S., Van Domselaar, G., Wu, J., Earn, D., & Ogden, N. H. (2021). The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Current biology : CB, 31(14), R918–R929. https://doi.org/10.1016/j.cub.2021.06.049